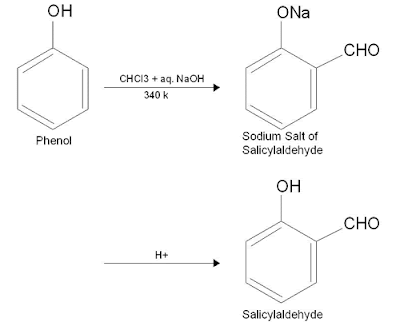
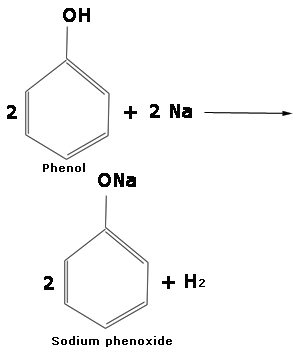
Prepration of phenol

Preparation of phenol from benzene derivatives Phenol was first isolated in the early nineteenth century from coal tar. Nowadays, phenol is commercially produced synthetically. In the laboratory, phenols may be prepared from benzene derivatives by any of the following methods. 1. From sodium benzene sulphonate Benezene sulphonic acid when treated with NaOH gives its sodium salt. Sodium benzene sulphonate. This when fused with NaOH at temperature between 570-620 K, gives sodium phenoxide, which on hydrolysis with dilute mineral acid gives phenol. 2. From Benzene diazonium chloride Benzene diazonium chloride is formed by treating aniline with nitrous acid (NANO2 + HCl) at 273-283 K temperature. On warming an aqueous solution of benzene diazonium chloride, it is hydrolysed to form phenol. 3. From Chlorobenzene (Dow’s process) Chlorobenzene on heating with 10% aqueous solution of NaOH at about 623K under 200 atmospheric pressure in the presence of copper salt catalyst, sodium phenoxide is ...