Reaction of alcohol and phenol with Metals
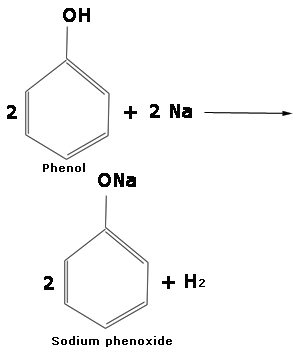
Alcohols and phenols react with highly electropositive alkali metals such as sodium, potassium etc to yield corresponding alkoxides/phenoxides and hydrogen.
2 R - OH + 2 Na ---------> 2 RONa (Sodium alkoxide) + H2
2 C2H5OH + 2 Na ----------> 2 C2H5ONa (Sodium ethoxide)+ H2
Related post Chemical properties of Alcohols and Phenols
2 R - OH + 2 Na ---------> 2 RONa (Sodium alkoxide) + H2
2 C2H5OH + 2 Na ----------> 2 C2H5ONa (Sodium ethoxide)+ H2

Related post Chemical properties of Alcohols and Phenols

Comments
Post a Comment