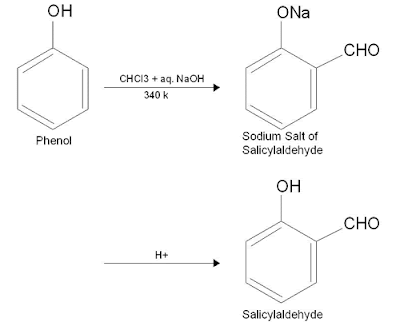
Reimer-Tiemann reaction of phenol
When phenol is heated with chloroform in presence of aqueous alkali (NaOH) at 340 K followed by hydrolysis of the resulting product a -CHO group is introduced at ortho position of benzene ring to form a phenolic aldehyde known as salicylaldehyde (O- hydroxy benzaldehyde) as the main product. A small amount of the para isomer is also obtained. This reaction is known as Reimer - Tiemann reaction.
Instead of chloroform, if carbon tetrachloride is used in the above reaction salicylic acid (O- hydroxy benzoic acid) is obtained as the major product.

The pictures of Reimer-Tiemann reaction of phenol will added soon.
ReplyDeleteIf you find any Suggestion or Error Please comment here.