Reaction of alcohol and phenol with alkali
Phenols react with aqueous sodium hydroxide to form sodium phenoxides. But alcohols are neutral to this reaction.

The above reactions show that alcohols and phenols are acidic in nature. In fact, alcohols and phenols are Bronsted acids, that is they can donate a proton to a strong base (B:)
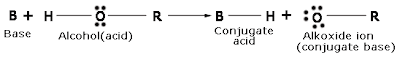
On treating an alkoxide with water the starting alcohol is obtained.

This reaction shows that water is a better proton donor than alcohol. In other words, alcohols are weaker acids than water. Also, in the above reaction, we can see that alkoxide ion is a better proton acceptor than hydroxide ion, which shows that alkoxides are stronger bases. For example, sodium ethoxide is a stronger base than sodium hydroxide.
The acidic character of alcohols is due to the polar O-H bond. An electron releasing group increases the electron density over the oxygen atom tending to decrease the polarity of O-H bond. This decreases the acid strength of alcohols decreases in the following order.

Obviously, the basic strength of their alkoxides follows the reverse order.
Alcohols acts as Bronsted bases as well. It is due to the presence of unshared electron pairs over oxygen, which makes alcohols proton aceptors.
Related article Chemical properties of Alcohols and Phenols

The above reactions show that alcohols and phenols are acidic in nature. In fact, alcohols and phenols are Bronsted acids, that is they can donate a proton to a strong base (B:)
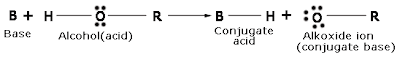
On treating an alkoxide with water the starting alcohol is obtained.

This reaction shows that water is a better proton donor than alcohol. In other words, alcohols are weaker acids than water. Also, in the above reaction, we can see that alkoxide ion is a better proton acceptor than hydroxide ion, which shows that alkoxides are stronger bases. For example, sodium ethoxide is a stronger base than sodium hydroxide.
The acidic character of alcohols is due to the polar O-H bond. An electron releasing group increases the electron density over the oxygen atom tending to decrease the polarity of O-H bond. This decreases the acid strength of alcohols decreases in the following order.

Obviously, the basic strength of their alkoxides follows the reverse order.
Alcohols acts as Bronsted bases as well. It is due to the presence of unshared electron pairs over oxygen, which makes alcohols proton aceptors.
Related article Chemical properties of Alcohols and Phenols

The concept of digital marketing has evolved to become a broader marketing strategy that involves the use of digital technologies, including mobile phones, social media, the internet, mobile applications, e-commerce, web analytics, and conversion optimization. We provide students with the opportunity to learn about the different ways they can use the internet for marketing products and services.
ReplyDeleteWe provide below Services:
Mental Health Nursing Assignment Help
Matlab Assignment Help
MATLAB Assignment Help Online
Joomla Assignment Expert
Labour Law Assignment Help