Oxides of Xenon ( XeO3 and XeO4)
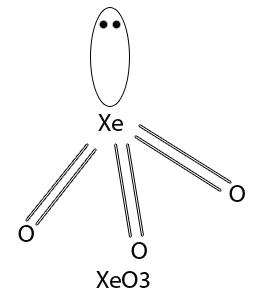
Xenon trioxide (XeO3)
XeO3 prepared by the slow hydrolysis of XeF6
XeF6 + 3H2O ------------> XeO3 + 6HF
Xenon trioxide is soluble in water and its aqueous solution is weakly acidic.
XeO3 + H2O <--------> H+ + HXeO4 – Xenate ion
XeO3 has pyramidal structrure in which Xe is in sp3 hybridisation.
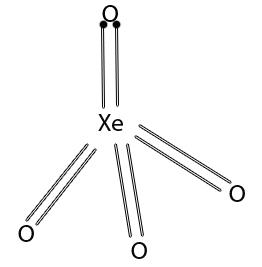
Xenon tetroxide (XeO4)
It is prepared by treating barium perxenate (Ba2XeO6) with anhydrous sulphuric acid.
Ba2XeO6 + 2H2SO4 -----------> XeO4 + 2BaSO4 + 2H2O
Xenon tetroxide is highly unstable and has tetrahedral structure.
Related article fluorides of xenon
For more detail visit indiastudychannel.com
XeO3 prepared by the slow hydrolysis of XeF6
XeF6 + 3H2O ------------> XeO3 + 6HF
Xenon trioxide is soluble in water and its aqueous solution is weakly acidic.
XeO3 + H2O <--------> H+ + HXeO4 – Xenate ion
XeO3 has pyramidal structrure in which Xe is in sp3 hybridisation.

Xenon tetroxide (XeO4)
It is prepared by treating barium perxenate (Ba2XeO6) with anhydrous sulphuric acid.
Ba2XeO6 + 2H2SO4 -----------> XeO4 + 2BaSO4 + 2H2O
Xenon tetroxide is highly unstable and has tetrahedral structure.

Related article fluorides of xenon
For more detail visit indiastudychannel.com

Comments
Post a Comment