Fluorides of xenon
The important fluorides of xenon are xenon difluoride(XeF2), Xenon tetrafluoride(Xef4) and xenon hexafluoride.
1. Xenon difluoride(XeF2)
It is prepared by heating a mixture of Xenon and fluorine in the ration 2:1 at 400 degree Celsius and 1 bar pressure in a sealed nickel tube.
Xe + F2 ---Ni----> XeF2
XeF2 undergoes hydrolysis when treated with water an d evolves oxygen.
2XeF2 + 2H2O -------> 2Xe + 4HF + O2
In XeF2, Xenon is sp3d hybridised and the molecule has linear structure as shown.
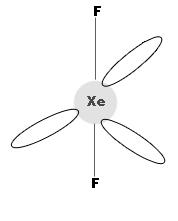
2. Xenon tetrafluoride (Xef4)
It is prepared by heating a mixture of Xe and F2 in the molecular ratio 1:5 at 400 degree Celsius and 6 atm in a sealed nickel tube.
Xe + 2 F2 ---------> XeF4
XeF4 react with water and produces explosive XeO3
6 XeF4 + 12 H2O ---------> 2 XeO3 + 4 Xe + 3O2 + 24 HF
In XeF4, Xenon is in sp3d2 hybridised state and has square planar geometry.
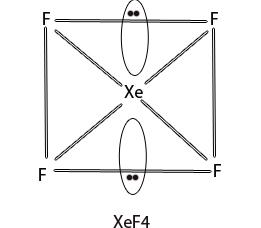
3. Xenon hexafluoride (XeF6)
It is prepared by heating a mixture of xenon and fluorine in the ration 1:20 at 300 degree Celsius and 60 atm in a nicked vessel.
Xe + 3 F2 --------> Xe F6
XeF6 Undergoes slow hydrolysis with atmospheric moisture producing highly explosive XeO3.
XeF6 + 3H2O --------> XeO3 + 6 HF
XeF6 molecule possesses distorted octahedral structure. The Xe atom in XeF6 is in sp3d3 hybridisation.
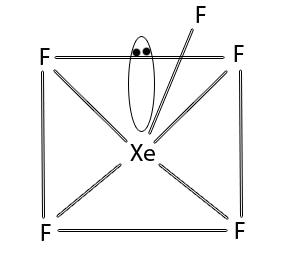
Related article Oxides of xenon
For more details visit indiastudyshannel.com
1. Xenon difluoride(XeF2)
It is prepared by heating a mixture of Xenon and fluorine in the ration 2:1 at 400 degree Celsius and 1 bar pressure in a sealed nickel tube.
Xe + F2 ---Ni----> XeF2
XeF2 undergoes hydrolysis when treated with water an d evolves oxygen.
2XeF2 + 2H2O -------> 2Xe + 4HF + O2
In XeF2, Xenon is sp3d hybridised and the molecule has linear structure as shown.

2. Xenon tetrafluoride (Xef4)
It is prepared by heating a mixture of Xe and F2 in the molecular ratio 1:5 at 400 degree Celsius and 6 atm in a sealed nickel tube.
Xe + 2 F2 ---------> XeF4
XeF4 react with water and produces explosive XeO3
6 XeF4 + 12 H2O ---------> 2 XeO3 + 4 Xe + 3O2 + 24 HF
In XeF4, Xenon is in sp3d2 hybridised state and has square planar geometry.

3. Xenon hexafluoride (XeF6)
It is prepared by heating a mixture of xenon and fluorine in the ration 1:20 at 300 degree Celsius and 60 atm in a nicked vessel.
Xe + 3 F2 --------> Xe F6
XeF6 Undergoes slow hydrolysis with atmospheric moisture producing highly explosive XeO3.
XeF6 + 3H2O --------> XeO3 + 6 HF
XeF6 molecule possesses distorted octahedral structure. The Xe atom in XeF6 is in sp3d3 hybridisation.

Related article Oxides of xenon
For more details visit indiastudyshannel.com

Comments
Post a Comment