Oxoacids of phosphorus
Phosphorus forms a number of oxoacids. They are hypophosphorous acid (H3PO2), Phosphorous acid (H3PO3), Hypophosphoric acid (H3PO4), pyrophosphoric acid (H4P2O7) and meta phosphoric acid (HPO2)n.
1. Hypo phosphorous acid (H3PO2) or phosphinic acid
It is preapred by the oxidation of phosphine by iodine in the presence of calculated amount of water. It is a monobasic acid.
PH3 + 2I2 + 2H2O -----------> H3PO2 + 4 HI
![]()
2. Phosphorous acid (H3PO3) or phosphonic acid
It is prepared by hydrolysis of phosphorous trioxide (P4O6). Phosphorous acid is dibasic.
P4O6 + 6H2O ----------> 4H3PO3

3. Hypophosphoric acid (H4P2O6)
It is prepared by controlled oxidation of red phosphorous with sodium chlorite solution, when disodium salt of hypophosphoric acid is formed which then passing through cation exchanger yield hypophosphoric acid. Hypophosphoric acid is tetrabasic.
2P + 2NaClO2 + 2H2O ---------> Na2H2P2O6 + 2HCl
Na2H2P2O6 + 2H -----resin-----> H4P2O6 + 2Na - resin

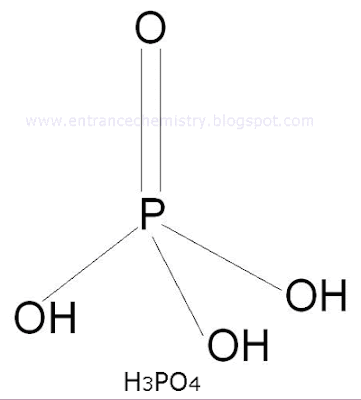
4. Orthophosphoric acid (H3PO4)
It is prepared by treating P4O10 with boiled water. It is a tri basic acid.
P4O10 + 6H2O ----------> 4H3PO4
5. Pyrophosphoric acid (H4p2O7)
It is prepared by heating orthophosphonic acid about 250oc. It is a tetrabasic acid.
2H3PO4 ---------> H4P2O7 + H2O

6. Meta phosphoric (HPO3)n.
It is obtained by heating orthophosphoric acid to about 850 K. Metaphosphoric acid does not exist as monomer. It exist as cyclic trimer, cyclic tetramer or polymer.
H3PO4 -------------> HPO3 + H2O
Related articles
1. Hypo phosphorous acid (H3PO2) or phosphinic acid
It is preapred by the oxidation of phosphine by iodine in the presence of calculated amount of water. It is a monobasic acid.
PH3 + 2I2 + 2H2O -----------> H3PO2 + 4 HI
2. Phosphorous acid (H3PO3) or phosphonic acid
It is prepared by hydrolysis of phosphorous trioxide (P4O6). Phosphorous acid is dibasic.
P4O6 + 6H2O ----------> 4H3PO3

3. Hypophosphoric acid (H4P2O6)
It is prepared by controlled oxidation of red phosphorous with sodium chlorite solution, when disodium salt of hypophosphoric acid is formed which then passing through cation exchanger yield hypophosphoric acid. Hypophosphoric acid is tetrabasic.
2P + 2NaClO2 + 2H2O ---------> Na2H2P2O6 + 2HCl
Na2H2P2O6 + 2H -----resin-----> H4P2O6 + 2Na - resin

4. Orthophosphoric acid (H3PO4)
It is prepared by treating P4O10 with boiled water. It is a tri basic acid.
P4O10 + 6H2O ----------> 4H3PO4

5. Pyrophosphoric acid (H4p2O7)
It is prepared by heating orthophosphonic acid about 250oc. It is a tetrabasic acid.
2H3PO4 ---------> H4P2O7 + H2O

6. Meta phosphoric (HPO3)n.
It is obtained by heating orthophosphoric acid to about 850 K. Metaphosphoric acid does not exist as monomer. It exist as cyclic trimer, cyclic tetramer or polymer.
H3PO4 -------------> HPO3 + H2O
Related articles
Oxides Of Phosphorus
Phosphine gas

Thanks
ReplyDeletevery nice post about oxyacids of phosphorous.
For important questions gk visit www.importantquestionsabout.blogspot.com