Dehydration of alcohol
Alcohols undergo dehydration (removal of a molecule of water) on treating them with protonic acids(conc. H2SO4 or H3PO4) or catalysts such as anhydrous zinc chloride or alumina to form alkenes.
Ethanol undergoes dehydration to form ethylene by heating it with concentrated H2SO4 at 443 K.
C2H5OH (ethanol)----(conc.H2SO4, 443 K)-----> CH2 = CH2(ethylene) + H2O
Secondary and tertiary alcohols are dehydrated under milder conditions.
For example


The relative ease of dehydration of alcohols follows the order:
Tertiary alcohol > Secondary alcohol > Primary alcohol
In the above reaction, if alcohol is in excess acidic dehydration takes place in a different way to yield an ether.
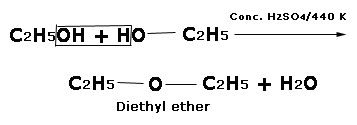
The ether is formed by the elimination of one water between molecule from two hydroxyl groups.
Related post Chemical properties of Alcohols and Phenols
Ethanol undergoes dehydration to form ethylene by heating it with concentrated H2SO4 at 443 K.
C2H5OH (ethanol)----(conc.H2SO4, 443 K)-----> CH2 = CH2(ethylene) + H2O
Secondary and tertiary alcohols are dehydrated under milder conditions.
For example


The relative ease of dehydration of alcohols follows the order:
Tertiary alcohol > Secondary alcohol > Primary alcohol
In the above reaction, if alcohol is in excess acidic dehydration takes place in a different way to yield an ether.
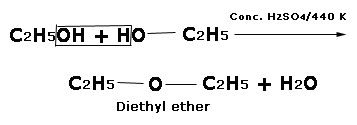
The ether is formed by the elimination of one water between molecule from two hydroxyl groups.
Related post Chemical properties of Alcohols and Phenols

Comments
Post a Comment